| ⇦ | 
| ⇨ |
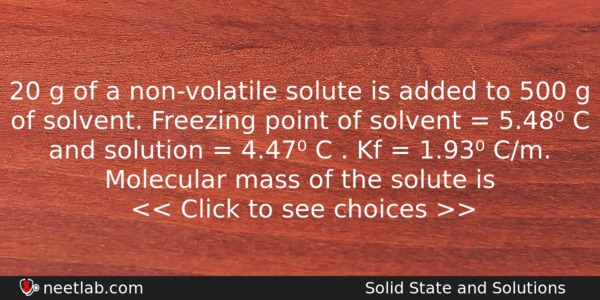
20 g of a non-volatile solute is added to 500 g of solvent. Freezing point of solvent = 5.48⁰ C and solution = 4.47⁰ C . Kf = 1.93⁰ C/m. Molecular mass of the solute is
Options
(a) 77.2
(b) 76.4
(c) 73.2
(d) 70.6
Correct Answer:
76.4
Explanation:
No explanation available. Be the first to write the explanation for this question by commenting below.
Related Questions: - Among these intensive property is
- KMnO₄ acts as an oxidising agent in the netural medium and gets reduced to MnO₂.
- The boiling point of water decreases at higher altitudes because
- The wavelength of the radiation emitted when an electron drops from 3rd orbit
- Phenol on reaction with bromine water would give
Topics: Solid State and Solutions
(91)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- Among these intensive property is
- KMnO₄ acts as an oxidising agent in the netural medium and gets reduced to MnO₂.
- The boiling point of water decreases at higher altitudes because
- The wavelength of the radiation emitted when an electron drops from 3rd orbit
- Phenol on reaction with bromine water would give
Topics: Solid State and Solutions (91)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply