| ⇦ | 
| ⇨ |
Ionization Enthalpy is the energy required to remove one electron from isolated gaseous atom in ground state. It is also called as Ionisation energy or Ionisation Potential.
[lyte id=”wydOJwCrU3k” \]
Ionization Enthalpy Trends in Periodic Table
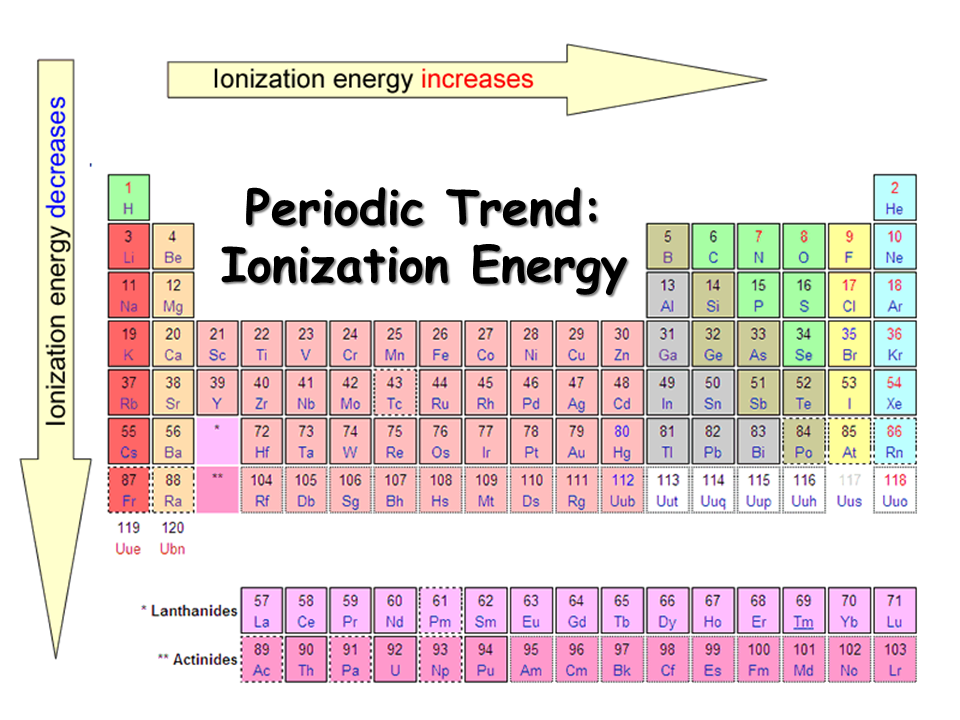
- Ist Ionization energy increases when we move from left to right across the period.
- Ist Ionization energy decreases as we go down the group.
Points to Remember
- Energy required to remove the first electron from the atom is called as Ist Ionization energy(I1). Energy required to remove the second electron from the atom is called as IInd Ionization energy(I2) and so on. This removal of electron happens till the atom reaches the completely filled state in the immediate inner shell.
- Levels of Ionization Energy
- I1 => (X → X+ + e−)
- I2 => (X+ → X2+ + e−)
- I3 => (X2+ → X3+ + e−)
- I1 < I2 < I3
- Ionization Energy is the opposite of Electron gain enthalpy.
Related Concepts
Electron Gain Enthalpy, Cation, Valence Electrons
Related Questions
- Which of the following elements will have the lowest first ionisation energy?
- When the first ionisation energies are plotted against atomic number
- The ionisation energy of nitrogoen is more than that of oxygen because
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply