| ⇦ | 
| ⇨ |
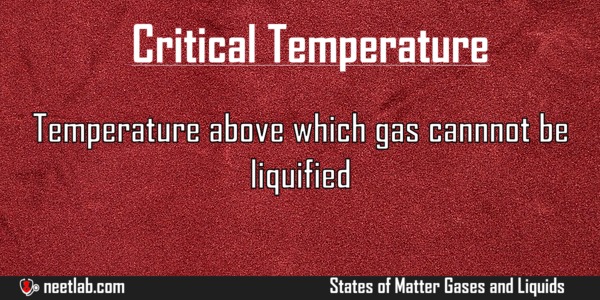
Temperature above which gas cannot be liquefied by pressure alone.
[lyte id=”OV0cDrEAE0k” \]
Points to Remember:
- Gases can be converted to liquids by compressing the gas at a suitable temperature.
- The critical temperature of a substance is the temperature at and above which vapour of the substance cannot be liquefied, no matter how much pressure is applied.
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply