| ⇦ | 
| ⇨ |
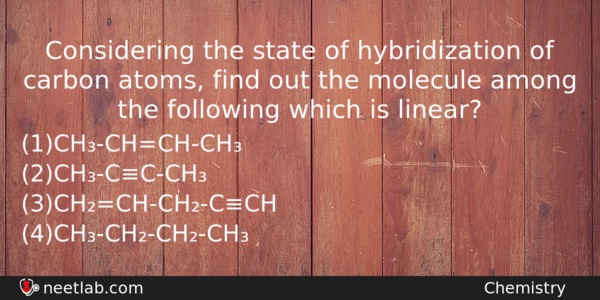
Considering the state of hybridization of carbon atoms, find out the molecule among the following which is linear?
Options
(a) CH₃-CH=CH-CH₃
(b) CH₃-C≡C-CH₃
(c) CH₂=CH-CH₂-C≡CH
(d) CH₃-CH₂-CH₂-CH₃
Correct Answer:
CH₃-C≡C-CH₃
Explanation:
sp³ sp sp sp³
H₃C – C ≡ C – CH₃
linear
Related Questions: - The IUPAC name of the compound having the formula CCl₃CH₂CHO is
- Which of the following isomeric alcohols react fastest with HCL
- Electrolytic reduction of nitrobenzene in weakly acidic medium gives
- How much energy is released when 6 moles of octane is burnt in air
- Which is the correct order of increasing energy of the listed orbitals in the atom
Question Type: Memory
(964)
Difficulty Level: Easy
(1008)
Topics: Chemical Bonding and Molecular Structure
(86)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- The IUPAC name of the compound having the formula CCl₃CH₂CHO is
- Which of the following isomeric alcohols react fastest with HCL
- Electrolytic reduction of nitrobenzene in weakly acidic medium gives
- How much energy is released when 6 moles of octane is burnt in air
- Which is the correct order of increasing energy of the listed orbitals in the atom
Question Type: Memory (964)
Difficulty Level: Easy (1008)
Topics: Chemical Bonding and Molecular Structure (86)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply