| ⇦ | 
| ⇨ |
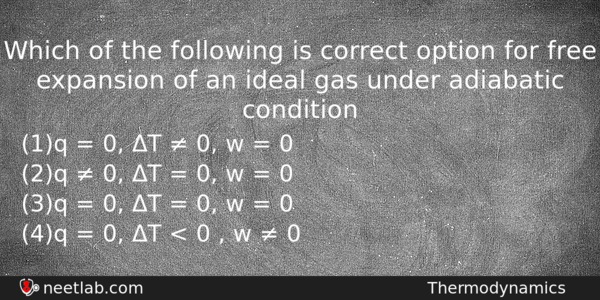
Which of the following is correct option for free expansion of an ideal gas under adiabatic condition
Options
(a) q = 0, ΔT ≠ 0, w = 0
(b) q ≠ 0, ΔT = 0, w = 0
(c) q = 0, ΔT = 0, w = 0
(d) q = 0, ΔT < 0 , w ≠ 0
Correct Answer:
q = 0, ΔT = 0, w = 0
Explanation:
For free expansion of an ideal gas under adiabatic condition, q = 0, ΔT = 0, w = 0.
Related Questions: - Excess of Na⁺ ions in air system causes
- What is the value of carbonate hardness of water sample if 100 mL of it took
- The weight of iron which will be converted into its oxide(Fe₃O₄) by the action of 18g
- The pH value of 10⁻⁸ M HCl is
- Propanone and propanal are …. Isomers
Topics: Thermodynamics
(179)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- Excess of Na⁺ ions in air system causes
- What is the value of carbonate hardness of water sample if 100 mL of it took
- The weight of iron which will be converted into its oxide(Fe₃O₄) by the action of 18g
- The pH value of 10⁻⁸ M HCl is
- Propanone and propanal are …. Isomers
Topics: Thermodynamics (179)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply