| ⇦ | 
| ⇨ |
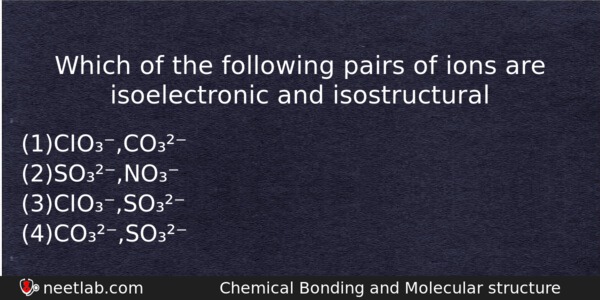
Which of the following pairs of ions are isoelectronic and isostructural
Options
(a) CIO₃⁻,CO₃²⁻
(b) SO₃²⁻,NO₃⁻
(c) CIO₃⁻,SO₃²⁻
(d) CO₃²⁻,SO₃²⁻
Correct Answer:
CIO₃⁻,SO₃²⁻
Explanation:
ClO₃⁻ and SO₃⁻ ² both have same number of electrons (42) and central atom in each being sp³ hybridised. Both are having one lone pair on central atom hence they are pyramidal.
Related Questions: - The maximum energy is possessed by an electrons,when it is present
- Solubility of MX₂ – type electrolytes is 0.5 ˣ 10⁻⁴ mol/L.,
- Reaction of a carbonyl compound with one of the following reagents involves
- A reaction occurs spontaneously is
- The correct order of stability of the superoxides is
Topics: Chemical Bonding and Molecular Structure
(86)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- The maximum energy is possessed by an electrons,when it is present
- Solubility of MX₂ – type electrolytes is 0.5 ˣ 10⁻⁴ mol/L.,
- Reaction of a carbonyl compound with one of the following reagents involves
- A reaction occurs spontaneously is
- The correct order of stability of the superoxides is
Topics: Chemical Bonding and Molecular Structure (86)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply