| ⇦ | 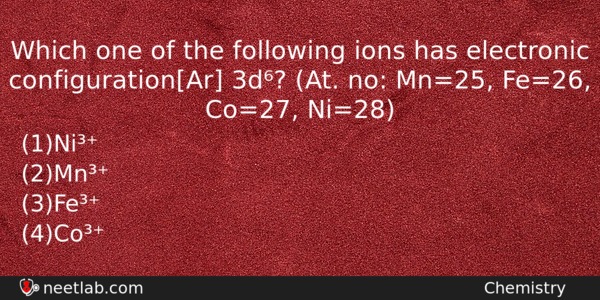
| ⇨ |
Which one of the following ions has electronic configuration[Ar] 3d⁶? (At. no: Mn=25, Fe=26, Co=27, Ni=28)
Options
(a) Ni³⁺
(b) Mn³⁺
(c) Fe³⁺
(d) Co³⁺
Correct Answer:
Co³⁺
Explanation:
Ni³⁺ (28) = [Ar] 3d⁷
Mn³⁺ (25) = [Ar] 3d⁴
Fe³⁺ (26) = [Ar] 3d⁵
Co³⁺ (27) = [Ar] 3d⁶
Related Questions: - Which of the following will not show resonance
- Phenylethyl ether when boiled with concentrated HBr gives
- The cell used for the electrolysis of fused NaCl is
- Kinetic energy of one mole of an ideal gas at 300 K is
- Among the following the paramagnetic one is
Question Type: Memory
(964)
Difficulty Level: Easy
(1008)
Topics: Structure of Atom
(90)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- Which of the following will not show resonance
- Phenylethyl ether when boiled with concentrated HBr gives
- The cell used for the electrolysis of fused NaCl is
- Kinetic energy of one mole of an ideal gas at 300 K is
- Among the following the paramagnetic one is
Question Type: Memory (964)
Difficulty Level: Easy (1008)
Topics: Structure of Atom (90)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply