| ⇦ | 
| ⇨ |
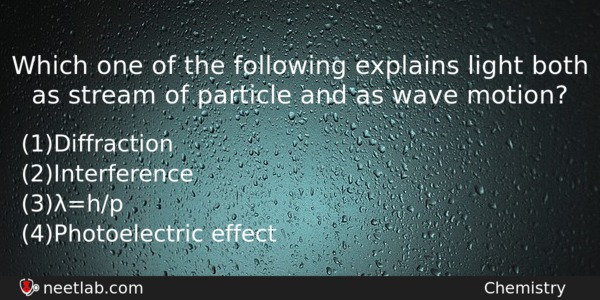
Which one of the following explains light both as stream of particle and as wave motion?
Options
(a) Diffraction
(b) Interference
(c) λ=h/p
(d) Photoelectric effect
Correct Answer:
λ=h/p
Explanation:
λ= h/p = h/mv = h/mc
Related Questions: - Polarisation power of a cation increases,when
- The equivalent weight of phosphoric acid(H₃PO₄) in the reaction
- The wrong statement about fullerene is
- The normality of solution containing 31.5g of hydrated oxalic acid (H₂C₂O₄.2H₂O)
- The smallest among the following ion is
Question Type: Memory
(964)
Difficulty Level: Easy
(1008)
Topics: Structure of Atom
(90)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- Polarisation power of a cation increases,when
- The equivalent weight of phosphoric acid(H₃PO₄) in the reaction
- The wrong statement about fullerene is
- The normality of solution containing 31.5g of hydrated oxalic acid (H₂C₂O₄.2H₂O)
- The smallest among the following ion is
Question Type: Memory (964)
Difficulty Level: Easy (1008)
Topics: Structure of Atom (90)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply