| ⇦ | 
| ⇨ |
Which one of the following esters gets hydrolysde most easily under alkaline conditions
Options
(a)
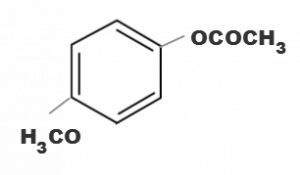
(b)
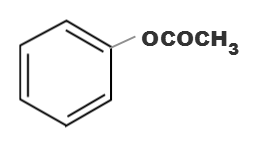
(c)
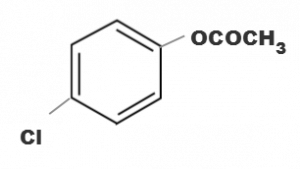
(d)
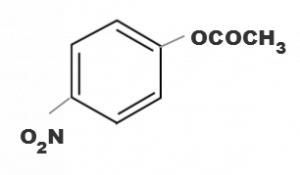
Correct Answer:
(d)

Explanation:
No explanation available. Be the first to write the explanation for this question by commenting below.
Related Questions: - Solubility of iodine in water may be increased by adding
- Naphthalene is a
- Which of the following explains the sequence of filling the electrons
- The increasing order of atomic radius for the elements Na, Rb, K and Mg is
- Which of the following oxides of nitrogen is paramagnetic
Topics: Aldehydes Ketones and Carboxylic Acid
(89)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- Solubility of iodine in water may be increased by adding
- Naphthalene is a
- Which of the following explains the sequence of filling the electrons
- The increasing order of atomic radius for the elements Na, Rb, K and Mg is
- Which of the following oxides of nitrogen is paramagnetic
Topics: Aldehydes Ketones and Carboxylic Acid (89)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

electron withdrawing group attatched to the benzene ring increases the reactivity towards nucleophilic substitution reaction. Since NO2 group is strong electron withdrawing group in basic medium.Hence ester containing NO2 group will be hydrolysed more easily