| ⇦ | 
| ⇨ |
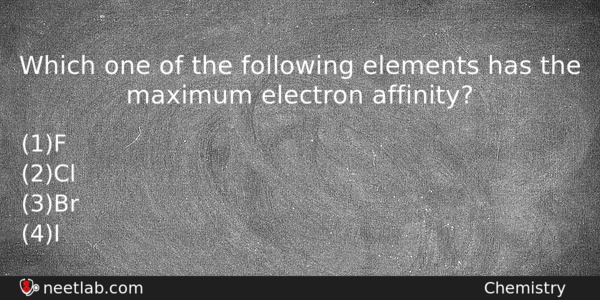
Which one of the following elements has the maximum electron affinity?
Options
(a) F
(b) Cl
(c) Br
(d) I
Correct Answer:
Cl
Explanation:
Chlorine hasmaximum electron affinity. Fluorine although have highest electronegativity dut to its very small size, effective inter electronic repulsions are observed which brings down its electron affinity.
Related Questions: - In the reaction of H₂O₂ with acidified KMnO₄
- Which one of the following is present as an active ingredient in bleaching
- Which one of the following reagents will be able to distinguish between 1-butyne
- Which kind of isomerism is exhibited by octahedral [Co(NH₃)₄Br₂]Cl
- Which among the following has the largest dipole moment
Question Type: Memory
(964)
Difficulty Level: Easy
(1008)
Topics: Classification of Elements and Periodicity
(94)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- In the reaction of H₂O₂ with acidified KMnO₄
- Which one of the following is present as an active ingredient in bleaching
- Which one of the following reagents will be able to distinguish between 1-butyne
- Which kind of isomerism is exhibited by octahedral [Co(NH₃)₄Br₂]Cl
- Which among the following has the largest dipole moment
Question Type: Memory (964)
Difficulty Level: Easy (1008)
Topics: Classification of Elements and Periodicity (94)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply