| ⇦ | 
| ⇨ |
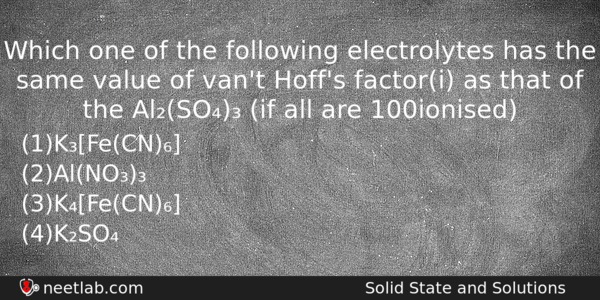
Which one of the following electrolytes has the same value of van’t Hoff’s factor(i) as that of the Al₂(SO₄)₃ (if all are 100% ionised)
Options
(a) K₃[Fe(CN)₆]
(b) Al(NO₃)₃
(c) K₄[Fe(CN)₆]
(d) K₂SO₄
Correct Answer:
K₄[Fe(CN)₆]
Explanation:
No explanation available. Be the first to write the explanation for this question by commenting below.
Related Questions: - The -OH group of an alcohol or the -COOH group of a carboxylic acid can be
- When 2-bromobutane reacts with alcoholic KOH, the reaction is called
- A corked flask containing boiling water and its vapour is allowed
- Which of the following compounds is isomeric with 2,2,4,4-tetramethylhexane
- IN Haber process 30 L of dihydrogen and 30 L of dinitrogen were taken for reaction
Topics: Solid State and Solutions
(91)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- The -OH group of an alcohol or the -COOH group of a carboxylic acid can be
- When 2-bromobutane reacts with alcoholic KOH, the reaction is called
- A corked flask containing boiling water and its vapour is allowed
- Which of the following compounds is isomeric with 2,2,4,4-tetramethylhexane
- IN Haber process 30 L of dihydrogen and 30 L of dinitrogen were taken for reaction
Topics: Solid State and Solutions (91)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply