| ⇦ | 
| ⇨ |
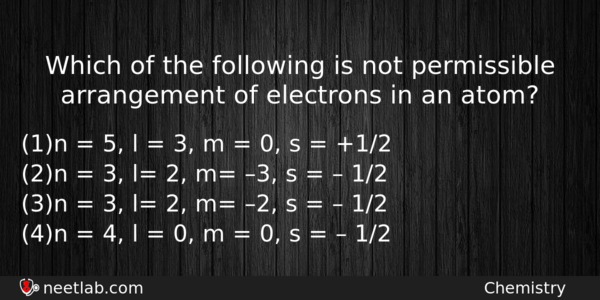
Which of the following is not permissible arrangement of electrons in an atom?
Options
(a) n = 5, l = 3, m = 0, s = +1/2
(b) n = 3, l= 2, m= –3, s = – 1/2
(c) n = 3, l= 2, m= –2, s = – 1/2
(d) n = 4, l = 0, m = 0, s = – 1/2
Correct Answer:
n = 3, l= 2, m= –3, s = – 1/2
Explanation:
m=2l+1, thus for l=2, m=5, hence values of m will be -2,-1,0,+1,+2. Therefore for l=2, m cannot have the value of -3
Related Questions: - Ethyl alcohol is soluble in water in all proportions, because it
- Which of the following order is wrong?
- Nitrobenzene combines with hydrogen in the presence of platinum to yield
- When chloroform is exposed to light and air, it forms
- Mg burns in CO₂ to produce
Question Type: Memory
(964)
Difficulty Level: Easy
(1008)
Topics: Structure of Atom
(90)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- Ethyl alcohol is soluble in water in all proportions, because it
- Which of the following order is wrong?
- Nitrobenzene combines with hydrogen in the presence of platinum to yield
- When chloroform is exposed to light and air, it forms
- Mg burns in CO₂ to produce
Question Type: Memory (964)
Difficulty Level: Easy (1008)
Topics: Structure of Atom (90)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply