| ⇦ | 
| ⇨ |
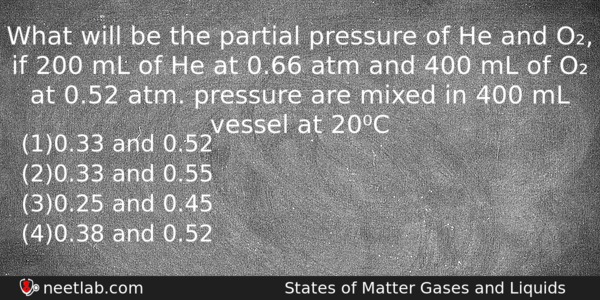
What will be the partial pressure of He and O₂, if 200 mL of He at 0.66 atm and 400 mL of O₂ at 0.52 atm. pressure are mixed in 400 mL vessel at 20⁰C
Options
(a) 0.33 and 0.52
(b) 0.33 and 0.55
(c) 0.25 and 0.45
(d) 0.38 and 0.52
Correct Answer:
0.33 and 0.52
Explanation:
Pʜₑ = (200 × 0.66) / 400 = 0.33 atm
Po₂ = (400 × 0.52) / 400 = 0.52 atm.
Related Questions: - In the silver plating of copper, K[Ag(CN)₂] is used instead of AgNO₃. The reason
- In the dichlorination reaction of propane, mixture of products are obtained.
- For an endothermic reaction, energy of activation is Ea and enthalpy of reaction
- Which of the following will be most stable diazonium salt RN₂⁺ X⁻
- Which of the following pairs of transition metal ions are the stronger oxidisi
Topics: States of Matter Gases and Liquids
(80)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- In the silver plating of copper, K[Ag(CN)₂] is used instead of AgNO₃. The reason
- In the dichlorination reaction of propane, mixture of products are obtained.
- For an endothermic reaction, energy of activation is Ea and enthalpy of reaction
- Which of the following will be most stable diazonium salt RN₂⁺ X⁻
- Which of the following pairs of transition metal ions are the stronger oxidisi
Topics: States of Matter Gases and Liquids (80)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply