| ⇦ | 
| ⇨ |
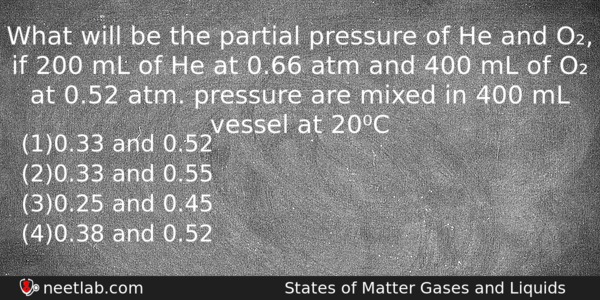
What will be the partial pressure of He and O₂, if 200 mL of He at 0.66 atm and 400 mL of O₂ at 0.52 atm. pressure are mixed in 400 mL vessel at 20⁰C
Options
(a) 0.33 and 0.52
(b) 0.33 and 0.55
(c) 0.25 and 0.45
(d) 0.38 and 0.52
Correct Answer:
0.33 and 0.52
Explanation:
Pʜₑ = (200 × 0.66) / 400 = 0.33 atm
Po₂ = (400 × 0.52) / 400 = 0.52 atm.
Related Questions: - According to IUPAC nomenclature sodium nitroprusside is named as
- In Gabriel phthalimide synthesis, the compound obtained is
- The smallest among the following ion is
- Neutral divalent carbon species released as reaction intermediate in reaction
- Equal volumes of 0.1 M AgNO₃ and 0.2 M NaCl are mixed
Topics: States of Matter Gases and Liquids
(80)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- According to IUPAC nomenclature sodium nitroprusside is named as
- In Gabriel phthalimide synthesis, the compound obtained is
- The smallest among the following ion is
- Neutral divalent carbon species released as reaction intermediate in reaction
- Equal volumes of 0.1 M AgNO₃ and 0.2 M NaCl are mixed
Topics: States of Matter Gases and Liquids (80)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply