| ⇦ | 
| ⇨ |
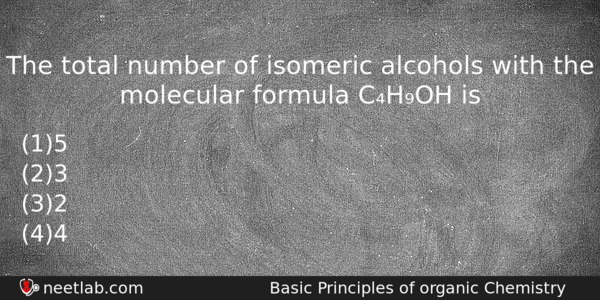
The total number of isomeric alcohols with the molecular formula C₄H₉OH is
Options
(a) 5
(b) 3
(c) 2
(d) 4
Correct Answer:
2
Explanation:
Molecular formula C₄H₉OH exists in two isomeric alcoholic forms, i.e., n-butyl alcohol and iso-butyl alcohol. therefore total number of isomeric alcohols is 2.
Related Questions: - Role of hydrogen peroxide in the above reactions is respectively
- The correct order of stability of the superoxides is
- Which of the following is always feasible
- What will be the partial pressure of He and O₂, if 200 mL of He at 0.66 atm and 400 mL
- The two electrons in K-subshell will differ in
Topics: Basic Principles of Organic Chemistry
(124)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- Role of hydrogen peroxide in the above reactions is respectively
- The correct order of stability of the superoxides is
- Which of the following is always feasible
- What will be the partial pressure of He and O₂, if 200 mL of He at 0.66 atm and 400 mL
- The two electrons in K-subshell will differ in
Topics: Basic Principles of Organic Chemistry (124)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply