| ⇦ | 
| ⇨ |
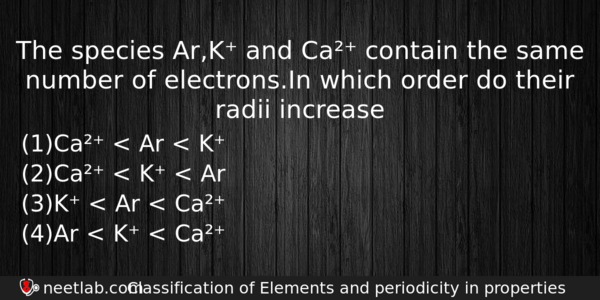
The species Ar,K⁺ and Ca²⁺ contain the same number of electrons.In which order do their radii increase
Options
(a) Ca²⁺ < Ar < K⁺
(b) Ca²⁺ < K⁺ < Ar
(c) K⁺ < Ar < Ca²⁺
(d) Ar < K⁺ < Ca²⁺
Correct Answer:
Ca²⁺ < K⁺ < Ar
Explanation:
In isoelectronic species the radius decrease with increase in nuclear charge hence increasing order of radius is Ca⁺² < K⁺< Ar
Related Questions: - Ziegler Natta catalyst is an organometallic compound containing
- Of the following, which change will shift the reaction towards the product
- An aqueous solution of CoCl₂ on addition of excess of concentration HCl
- Starch is converted into maltose by an enzyme
- Last molecule of H₂O is evolved from H₂O₂ by
Topics: Classification of Elements and Periodicity
(94)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- Ziegler Natta catalyst is an organometallic compound containing
- Of the following, which change will shift the reaction towards the product
- An aqueous solution of CoCl₂ on addition of excess of concentration HCl
- Starch is converted into maltose by an enzyme
- Last molecule of H₂O is evolved from H₂O₂ by
Topics: Classification of Elements and Periodicity (94)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply