| ⇦ | 
| ⇨ |
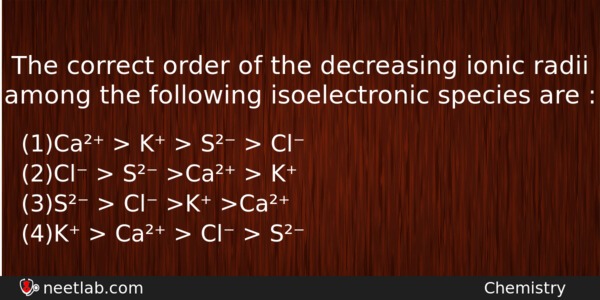
The correct order of the decreasing ionic radii among the following isoelectronic species are :
Options
(a) Ca²⁺ > K⁺ > S²⁻ > Cl⁻
(b) Cl⁻ > S²⁻ >Ca²⁺ > K⁺
(c) S²⁻ > Cl⁻ >K⁺ >Ca²⁺
(d) K⁺ > Ca²⁺ > Cl⁻ > S²⁻
Correct Answer:
S²⁻ > Cl⁻ >K⁺ >Ca²⁺
Explanation:
Among the isoelectronic species, size increases with the increase in negative charge. Thus S has the highest negative charge and hence largest in size followed by Cl, K and Ca
Related Questions: - If N and S both are present in an organic compound then during Lassaigne’s test,
- If 900 J/g of heat is exchanged at boiling point of water, then what is increase
- The Ca²⁺ and F⁻ are located in CaF₂ crystal,respectively at face centred cubic lattic points
- Picric acid is
- 0.4g of silver salt of a monobasic organic acid gave 0.26g pure silver on ignition
Question Type: Memory
(964)
Difficulty Level: Easy
(1008)
Topics: Classification of Elements and Periodicity
(94)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- If N and S both are present in an organic compound then during Lassaigne’s test,
- If 900 J/g of heat is exchanged at boiling point of water, then what is increase
- The Ca²⁺ and F⁻ are located in CaF₂ crystal,respectively at face centred cubic lattic points
- Picric acid is
- 0.4g of silver salt of a monobasic organic acid gave 0.26g pure silver on ignition
Question Type: Memory (964)
Difficulty Level: Easy (1008)
Topics: Classification of Elements and Periodicity (94)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply