| ⇦ | 
| ⇨ |
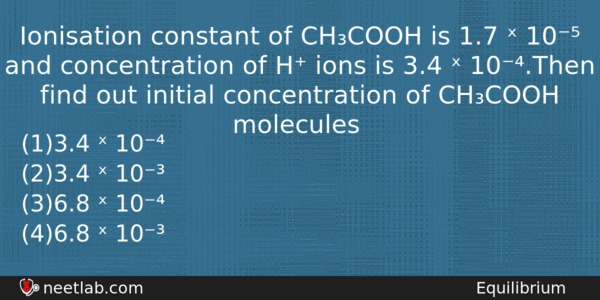
Ionisation constant of CH₃COOH is 1.7 ˣ 10⁻⁵ and concentration of H⁺ ions is 3.4 ˣ 10⁻⁴.Then find out initial concentration of CH₃COOH molecules
Options
(a) 3.4 ˣ 10⁻⁴
(b) 3.4 ˣ 10⁻³
(c) 6.8 ˣ 10⁻⁴
(d) 6.8 ˣ 10⁻³
Correct Answer:
6.8 ˣ 10⁻³
Explanation:
No explanation available. Be the first to write the explanation for this question by commenting below.
Related Questions: - If increase in temperature and volume of an ideal gas is two times, then the initial
- Chlorination of toluene in the presence of light and heat followed by treatment
- When conc. H₂SO₄ is added to dry KNO₃,brown fumes are evolved.These fumes are
- A nucleophile must have
- Which of the following has ability to release bromine from potassium bromide
Topics: Equilibrium
(104)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- If increase in temperature and volume of an ideal gas is two times, then the initial
- Chlorination of toluene in the presence of light and heat followed by treatment
- When conc. H₂SO₄ is added to dry KNO₃,brown fumes are evolved.These fumes are
- A nucleophile must have
- Which of the following has ability to release bromine from potassium bromide
Topics: Equilibrium (104)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply