| ⇦ | 
| ⇨ |
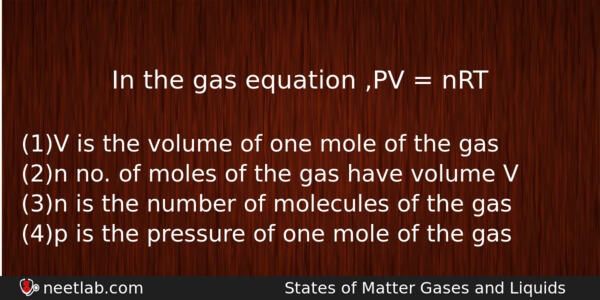
In the gas equation ,PV = nRT
Options
(a) V is the volume of one mole of the gas
(b) n no. of moles of the gas have volume V
(c) n is the number of molecules of the gas
(d) p is the pressure of one mole of the gas
Correct Answer:
n is the number of molecules of the gas
Explanation:
Ideal gas equation is : PV = nRT. P = Pressure of the gas. n = no.of molecules of gas . T = temperature. V = volume of gas. R = gas constant
Related Questions: - The reaction CH₃ – Br + 2Na + Br – CH₃ → CH₃ – CH₃ is called
- Which of the following statements is true for the electrochemical Daniel cell
- In the reaction with HCl, an alkene reacts in accordance with the Markovnikov’s
- The boiling point of water decreases at higher altitudes because
- Which of the following is correct option for free expansion of an ideal gas
Topics: States of Matter Gases and Liquids
(80)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- The reaction CH₃ – Br + 2Na + Br – CH₃ → CH₃ – CH₃ is called
- Which of the following statements is true for the electrochemical Daniel cell
- In the reaction with HCl, an alkene reacts in accordance with the Markovnikov’s
- The boiling point of water decreases at higher altitudes because
- Which of the following is correct option for free expansion of an ideal gas
Topics: States of Matter Gases and Liquids (80)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply