| ⇦ | 
| ⇨ |
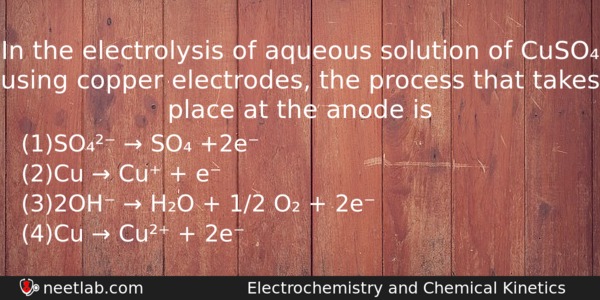
In the electrolysis of aqueous solution of CuSO₄ using copper electrodes, the process that takes place at the anode is
Options
(a) SO₄²⁻ → SO₄ +2e⁻
(b) Cu → Cu⁺ + e⁻
(c) 2OH⁻ → H₂O + 1/2 O₂ + 2e⁻
(d) Cu → Cu²⁺ + 2e⁻
Correct Answer:
Cu → Cu²⁺ + 2e⁻
Explanation:
No explanation available. Be the first to write the explanation for this question by commenting below.
Related Questions: - If 10 cm³ solution of H₂O₂ ,on decomposition gives 150 cm³ of O₂
- If an atom is reduced, its oxidation number
- The neutralisation of a strong acid by a strong base liberates an amount
- Alum in dyeing of clothes is used as
- When alkyl halide is heated, with dry Ag₂O, it produces
Topics: Electrochemistry and Chemical Kinetics
(87)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- If 10 cm³ solution of H₂O₂ ,on decomposition gives 150 cm³ of O₂
- If an atom is reduced, its oxidation number
- The neutralisation of a strong acid by a strong base liberates an amount
- Alum in dyeing of clothes is used as
- When alkyl halide is heated, with dry Ag₂O, it produces
Topics: Electrochemistry and Chemical Kinetics (87)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply