| ⇦ | 
| ⇨ |
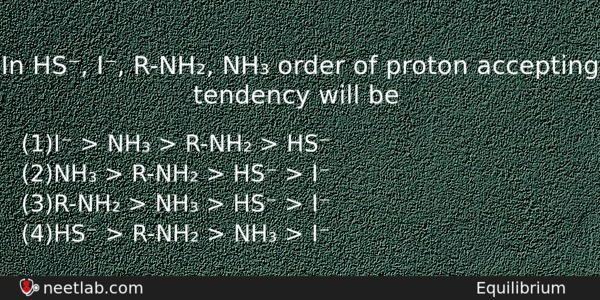
In HS⁻, I⁻, R-NH₂, NH₃ order of proton accepting tendency will be
Options
(a) I⁻ > NH₃ > R-NH₂ > HS⁻
(b) NH₃ > R-NH₂ > HS⁻ > I⁻
(c) R-NH₂ > NH₃ > HS⁻ > I⁻
(d) HS⁻ > R-NH₂ > NH₃ > I⁻
Correct Answer:
R-NH₂ > NH₃ > HS⁻ > I⁻
Explanation:
No explanation available. Be the first to write the explanation for this question by commenting below.
Related Questions: - In the cyanide ion, the formal negative charge is on
- The unit of ionic mobility is
- Carbogen is
- Which one of the following is not true for the hydrolysis of t-butyl bromide
- Average molar kinetic energy of CO and N₂ at same temperature is
Topics: Equilibrium
(104)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- In the cyanide ion, the formal negative charge is on
- The unit of ionic mobility is
- Carbogen is
- Which one of the following is not true for the hydrolysis of t-butyl bromide
- Average molar kinetic energy of CO and N₂ at same temperature is
Topics: Equilibrium (104)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply