| ⇦ | 
| ⇨ |
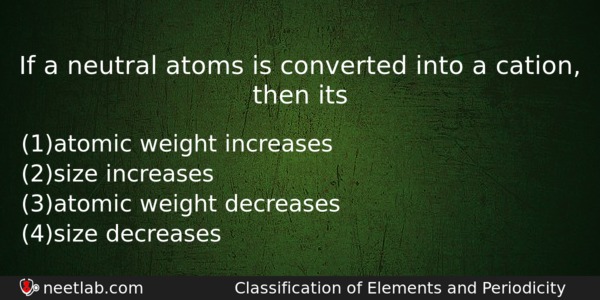
If a neutral atoms is converted into a cation, then its
Options
(a) atomic weight increases
(b) size increases
(c) atomic weight decreases
(d) size decreases
Correct Answer:
size decreases
Explanation:
After the removal of electron, to form cation, there is more effective nuclear charge on remaining electrons in the cation, so size decreases.
Related Questions: - If pressure is constant, the rate of diffusion varies with … proportional to density
- Which of the following has highest hydration energy
- The number of sigma and pi-bonds in but-1-ene-3-yne are
- The waxes are long chain compounds of fatty acids, which belong to the class of
- If 900 J/g of heat is exchanged at boiling point of water, then what is increase
Topics: Classification of Elements and Periodicity
(94)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- If pressure is constant, the rate of diffusion varies with … proportional to density
- Which of the following has highest hydration energy
- The number of sigma and pi-bonds in but-1-ene-3-yne are
- The waxes are long chain compounds of fatty acids, which belong to the class of
- If 900 J/g of heat is exchanged at boiling point of water, then what is increase
Topics: Classification of Elements and Periodicity (94)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply