| ⇦ | 
| ⇨ |
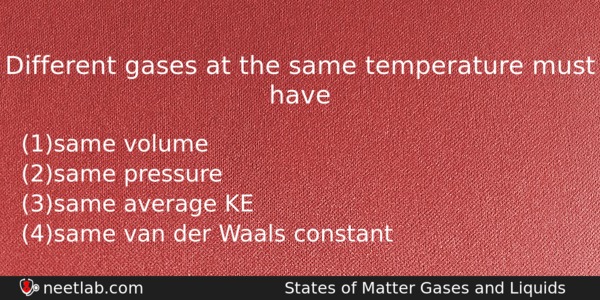
Different gases at the same temperature must have
Options
(a) same volume
(b) same pressure
(c) same average KE
(d) same van der Waals constant
Correct Answer:
same average KE
Explanation:
No explanation available. Be the first to write the explanation for this question by commenting below.
Related Questions: - The products formed when diborane is hydrolysed are
- If 300 mL of a gas at 27⁰ C is cooled to 7⁰C at constant pressure, its final volume
- A compound gives a positive test with I₂/NaOH and is extracted from benzene
- Which combination is best explained by the coordinate covalent bond?
- A liquid is in equilibrium with its vapour at its boiling point
Topics: States of Matter Gases and Liquids
(80)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- The products formed when diborane is hydrolysed are
- If 300 mL of a gas at 27⁰ C is cooled to 7⁰C at constant pressure, its final volume
- A compound gives a positive test with I₂/NaOH and is extracted from benzene
- Which combination is best explained by the coordinate covalent bond?
- A liquid is in equilibrium with its vapour at its boiling point
Topics: States of Matter Gases and Liquids (80)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply