| ⇦ | 
| ⇨ |
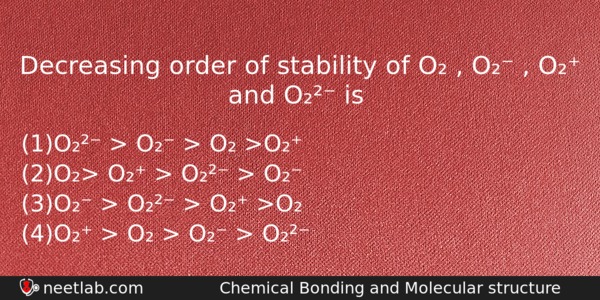
Decreasing order of stability of O₂ , O₂⁻ , O₂⁺ and O₂²⁻ is
Options
(a) O₂²⁻ > O₂⁻ > O₂ >O₂⁺
(b) O₂> O₂⁺ > O₂²⁻ > O₂⁻
(c) O₂⁻ > O₂²⁻ > O₂⁺ >O₂
(d) O₂⁺ > O₂ > O₂⁻ > O₂²⁻
Correct Answer:
O₂⁺ > O₂ > O₂⁻ > O₂²⁻
Explanation:
According to molecular orbital theory as bond order decreases stability of the molecule decreases
Bond order =1/2 (N(b)-N(a))
Bond order for O₂⁺=1/2 (10-5)=2.5
Bond order for O₂=1/2 (10-6)=2
Bond order for O₂⁻=1/2 (10-7)=1.5
Bond order for O₂²⁻=1/2 (10-8)=1.0
hence the correct order is
O₂⁺ > O₂ > O₂⁻> O₂²⁻
Related Questions: - The pair of compounds that can exist together is
- Temperature coefficient of a reaction is 2. When temperature is increased
- 3-Pentanol upon reaction with PBr₃ gives 2 and 3-bromopentane.Such rearrangement
- Siver chloride is soluble in methylamine due to the formation of
- In the reaction with HCl, an alkene reacts in accordance with the Markovnikov’s
Topics: Chemical Bonding and Molecular Structure
(86)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- The pair of compounds that can exist together is
- Temperature coefficient of a reaction is 2. When temperature is increased
- 3-Pentanol upon reaction with PBr₃ gives 2 and 3-bromopentane.Such rearrangement
- Siver chloride is soluble in methylamine due to the formation of
- In the reaction with HCl, an alkene reacts in accordance with the Markovnikov’s
Topics: Chemical Bonding and Molecular Structure (86)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply