| ⇦ | 
| ⇨ |
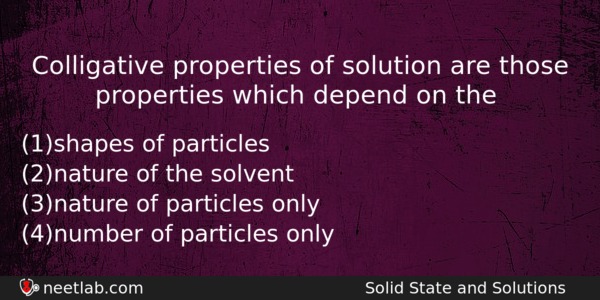
Colligative properties of solution are those properties which depend on the
Options
(a) shapes of particles
(b) nature of the solvent
(c) nature of particles only
(d) number of particles only
Correct Answer:
number of particles only
Explanation:
No explanation available. Be the first to write the explanation for this question by commenting below.
Related Questions: - When x g of magnesium burnt with oxygen what is left
- Diffusion of solvent through a semi permeable membrane is called
- The number of spherical nodes in 3p orbitals are
- For isothermal change in the case of an ideal gas
- H₂S acts only as a reducing agent while SO₂, can act both as a reducing
Topics: Solid State and Solutions
(91)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- When x g of magnesium burnt with oxygen what is left
- Diffusion of solvent through a semi permeable membrane is called
- The number of spherical nodes in 3p orbitals are
- For isothermal change in the case of an ideal gas
- H₂S acts only as a reducing agent while SO₂, can act both as a reducing
Topics: Solid State and Solutions (91)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply