| ⇦ | 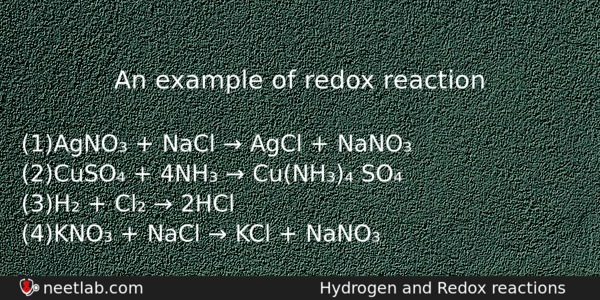
| ⇨ |
An example of redox reaction
Options
(a) AgNO₃ + NaCl → AgCl + NaNO₃
(b) CuSO₄ + 4NH₃ → Cu(NH₃)₄ SO₄
(c) H₂ + Cl₂ → 2HCl
(d) KNO₃ + NaCl → KCl + NaNO₃
Correct Answer:
H₂ + Cl₂ → 2HCl
Explanation:
H₂ + Cl₂ → 2HCl. H₂ (oxidation state = 0 ) changes to H⁺ (oxidation state = +1) and Cl₂ (oxidation state = 0) changes to Cl⁻ (oxidation state = -1). So H₂ has been oxidised and Cl₂ has been reduced, making this is redoxreaction.
Related Questions: - Phenol is heated with pthalic anhydride in the presence of concentrated H₂SO₄.
- In a buffer solution containing equal concentration of B⁻ and HB,
- The screening effect of d-electrons is
- The s-block element used as a catalyst in the manufacture of Buna-S rubber is
- Nitrobenzene, on nitration,produces
Topics: Hydrogen and Redox Reactions
(174)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- Phenol is heated with pthalic anhydride in the presence of concentrated H₂SO₄.
- In a buffer solution containing equal concentration of B⁻ and HB,
- The screening effect of d-electrons is
- The s-block element used as a catalyst in the manufacture of Buna-S rubber is
- Nitrobenzene, on nitration,produces
Topics: Hydrogen and Redox Reactions (174)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply