| ⇦ | 
| ⇨ |
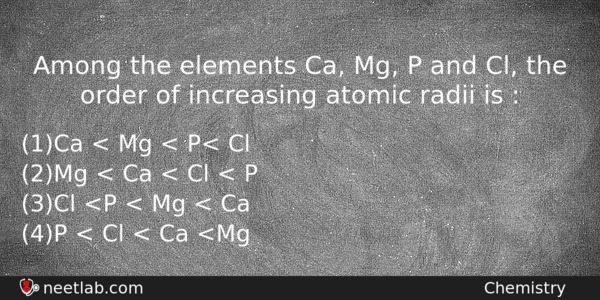
Among the elements Ca, Mg, P and Cl, the order of increasing atomic radii is :
Options
(a) Ca < Mg < P< Cl
(b) Mg < Ca < Cl < P
(c) Cl
Correct Answer:
Cl
Explanation:
No explanation available. Be the first to write the explanation for this question by commenting below.
Related Questions: - Which of the following polymers is hard
- A solid compound XY has NaCl structure . If the radius of the cation is 100 pm,
- MnO₃ in an acidic medium dissociates into
- Mutarotation does not occur in
- Lucas test is used for the determination of
Question Type: Memory
(964)
Difficulty Level: Easy
(1008)
Topics: Classification of Elements and Periodicity
(94)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- Which of the following polymers is hard
- A solid compound XY has NaCl structure . If the radius of the cation is 100 pm,
- MnO₃ in an acidic medium dissociates into
- Mutarotation does not occur in
- Lucas test is used for the determination of
Question Type: Memory (964)
Difficulty Level: Easy (1008)
Topics: Classification of Elements and Periodicity (94)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply