| ⇦ | 
| ⇨ |
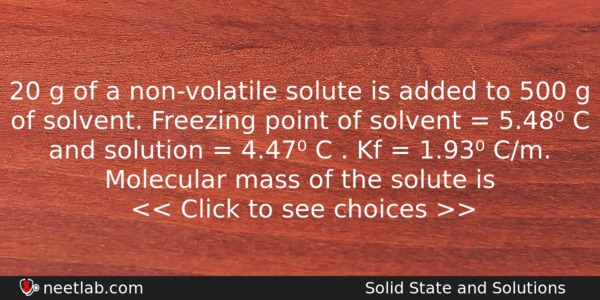
20 g of a non-volatile solute is added to 500 g of solvent. Freezing point of solvent = 5.48⁰ C and solution = 4.47⁰ C . Kf = 1.93⁰ C/m. Molecular mass of the solute is
Options
(a) 77.2
(b) 76.4
(c) 73.2
(d) 70.6
Correct Answer:
76.4
Explanation:
No explanation available. Be the first to write the explanation for this question by commenting below.
Related Questions: - Two moles of an ideal gas expand spontaneously into a vacuum. The work done is
- 0.45 g acid of molecular weight 90 was neutralise by 20 mL of 0.5 N KOH
- The calorific value of fats,carbohydrates and proteins is in the order of
- In the periodic table from left to right in a period,the atomic volume
- Ammonia can be dried by
Topics: Solid State and Solutions
(91)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- Two moles of an ideal gas expand spontaneously into a vacuum. The work done is
- 0.45 g acid of molecular weight 90 was neutralise by 20 mL of 0.5 N KOH
- The calorific value of fats,carbohydrates and proteins is in the order of
- In the periodic table from left to right in a period,the atomic volume
- Ammonia can be dried by
Topics: Solid State and Solutions (91)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply