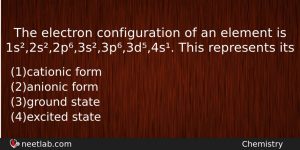
The electron configuration of an element is 1s²,2s²,2p⁶,3s²,3p⁶,3d⁵,4s¹
The electron configuration of an element is 1s²,2s²,2p⁶,3s²,3p⁶,3d⁵,4s¹. This represents its
Options
(a) cationic form (b) anionic form (c) ground state (d) excited state
Correct Answer:
ground state
Explanation:
This electron configuration is […]