| ⇦ | 
| ⇨ |
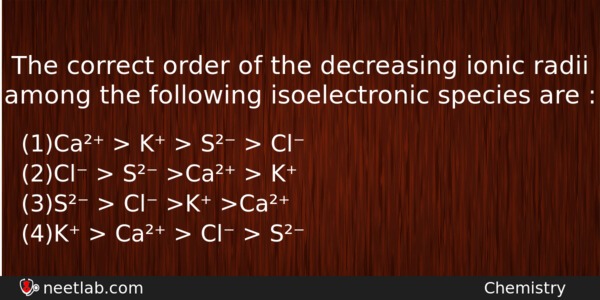
The correct order of the decreasing ionic radii among the following isoelectronic species are :
Options
(a) Ca²⁺ > K⁺ > S²⁻ > Cl⁻
(b) Cl⁻ > S²⁻ >Ca²⁺ > K⁺
(c) S²⁻ > Cl⁻ >K⁺ >Ca²⁺
(d) K⁺ > Ca²⁺ > Cl⁻ > S²⁻
Correct Answer:
S²⁻ > Cl⁻ >K⁺ >Ca²⁺
Explanation:
Among the isoelectronic species, size increases with the increase in negative charge. Thus S has the highest negative charge and hence largest in size followed by Cl, K and Ca
Related Questions: - The hardness of transition elements is due to their
- The fcc crystal contains how many atoms in each unit cell
- An atom has electronic configuration 1s²2s²2p⁶3s²3p⁶3d³4s², you will place it in
- Which does not exist
- Which of the following protein destroy the antigen when it enters in body call
Question Type: Memory
(964)
Difficulty Level: Easy
(1008)
Topics: Classification of Elements and Periodicity
(94)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- The hardness of transition elements is due to their
- The fcc crystal contains how many atoms in each unit cell
- An atom has electronic configuration 1s²2s²2p⁶3s²3p⁶3d³4s², you will place it in
- Which does not exist
- Which of the following protein destroy the antigen when it enters in body call
Question Type: Memory (964)
Difficulty Level: Easy (1008)
Topics: Classification of Elements and Periodicity (94)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply