| ⇦ | 
| ⇨ |
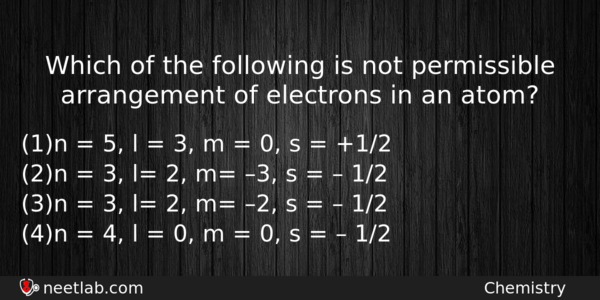
Which of the following is not permissible arrangement of electrons in an atom?
Options
(a) n = 5, l = 3, m = 0, s = +1/2
(b) n = 3, l= 2, m= –3, s = – 1/2
(c) n = 3, l= 2, m= –2, s = – 1/2
(d) n = 4, l = 0, m = 0, s = – 1/2
Correct Answer:
n = 3, l= 2, m= –3, s = – 1/2
Explanation:
m=2l+1, thus for l=2, m=5, hence values of m will be -2,-1,0,+1,+2. Therefore for l=2, m cannot have the value of -3
Related Questions: - The property,which can be classified as an intensive property, is
- By passing air over red hot coke the gas obtained is
- The wavelength of the radiation emitted when an electron drops from 3rd orbit
- Strong reducing behaviour of H₃PO₂ is due to
- Term ‘Hexadecane’ in petroleum is commonly called as
Question Type: Memory
(964)
Difficulty Level: Easy
(1008)
Topics: Structure of Atom
(90)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- The property,which can be classified as an intensive property, is
- By passing air over red hot coke the gas obtained is
- The wavelength of the radiation emitted when an electron drops from 3rd orbit
- Strong reducing behaviour of H₃PO₂ is due to
- Term ‘Hexadecane’ in petroleum is commonly called as
Question Type: Memory (964)
Difficulty Level: Easy (1008)
Topics: Structure of Atom (90)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply