| ⇦ | 
| ⇨ |
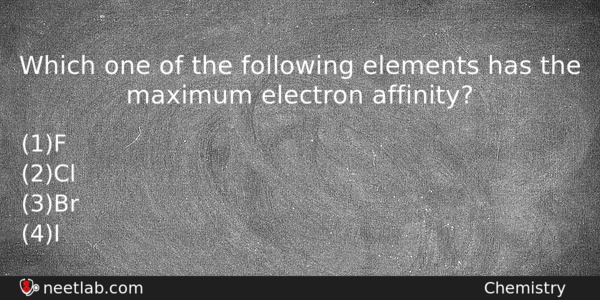
Which one of the following elements has the maximum electron affinity?
Options
(a) F
(b) Cl
(c) Br
(d) I
Correct Answer:
Cl
Explanation:
Chlorine hasmaximum electron affinity. Fluorine although have highest electronegativity dut to its very small size, effective inter electronic repulsions are observed which brings down its electron affinity.
Related Questions: - Number of possible isomers for the complex [Co(en)₂Cl₂]Cl
- Fibre reactive dyes are the derivatives of
- A solid compound X on heating gives CO₂ and a residue .
- Which of the following aqueous acid is most acidic
- Amino acids,Which build up proteins, have both the carboxylic and amino groups
Question Type: Memory
(964)
Difficulty Level: Easy
(1008)
Topics: Classification of Elements and Periodicity
(94)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- Number of possible isomers for the complex [Co(en)₂Cl₂]Cl
- Fibre reactive dyes are the derivatives of
- A solid compound X on heating gives CO₂ and a residue .
- Which of the following aqueous acid is most acidic
- Amino acids,Which build up proteins, have both the carboxylic and amino groups
Question Type: Memory (964)
Difficulty Level: Easy (1008)
Topics: Classification of Elements and Periodicity (94)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply