| ⇦ | 
| ⇨ |
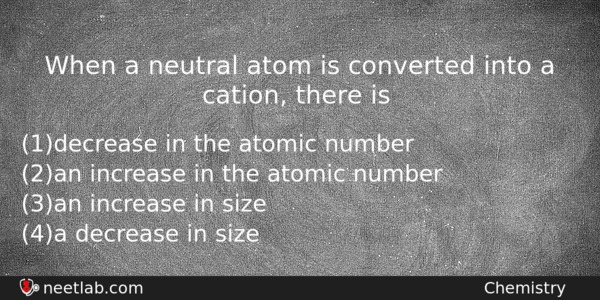
When a neutral atom is converted into a cation, there is
Options
(a) decrease in the atomic number
(b) an increase in the atomic number
(c) an increase in size
(d) a decrease in size
Correct Answer:
a decrease in size
Explanation:
During the conversion of a oneoutral atom into a cation, size decreases because after removal of one or more electron
(i) Nuclear charge per electron increases.
(ii) Outermost shell is completely removed.
Related Questions: - Strong reducing behaviour of H₃PO₂ is due to
- By which of the following processes permanent hardness of water can be removed
- When KMnO₄ reacts with KBr in alkaline medium gives bromate ion. Then oxidation
- How many unpaired electrons are present in ground state for Fe²⁺(Z=26)
- The boiling point of 0.2 mol kg⁻¹ solution of X in water is greater than equimolal
Question Type: Memory
(964)
Difficulty Level: Easy
(1008)
Topics: Classification of Elements and Periodicity
(94)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- Strong reducing behaviour of H₃PO₂ is due to
- By which of the following processes permanent hardness of water can be removed
- When KMnO₄ reacts with KBr in alkaline medium gives bromate ion. Then oxidation
- How many unpaired electrons are present in ground state for Fe²⁺(Z=26)
- The boiling point of 0.2 mol kg⁻¹ solution of X in water is greater than equimolal
Question Type: Memory (964)
Difficulty Level: Easy (1008)
Topics: Classification of Elements and Periodicity (94)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply