| ⇦ | 
| ⇨ |
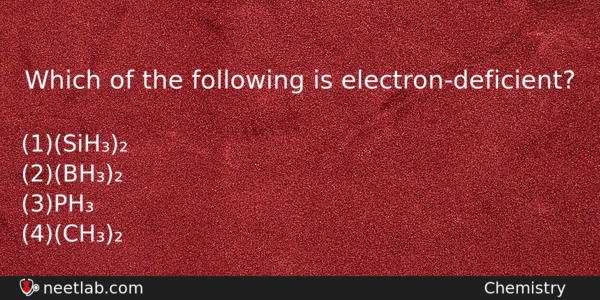
Which of the following is electron-deficient?
Options
(a) (SiH₃)₂
(b) (BH₃)₂
(c) PH₃
(d) (CH₃)₂
Correct Answer:
(BH₃)₂
Explanation:
Electron deficient molecules are compounds which do not have sufficient number of eletrons to form normal covalent bonds. (BH₃)₂ has two 3 centre – 2 electron bonds.
Related Questions: - In the process of nitration, the electrophile formed is
- Which of the following statement concerning lanthanides elements is fals
- If KMnO₄ is reduced by oxalic caid, in an acidic medium, then oxidation number of Mn changes from
- Which of the following is the green coloured powder produced when ammonium
- A zinc rod is place in 0.095 M solution of zinc sulphate at 298 K, the potential
Question Type: Memory
(964)
Difficulty Level: Easy
(1008)
Topics: Chemical Bonding and Molecular Structure
(86)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- In the process of nitration, the electrophile formed is
- Which of the following statement concerning lanthanides elements is fals
- If KMnO₄ is reduced by oxalic caid, in an acidic medium, then oxidation number of Mn changes from
- Which of the following is the green coloured powder produced when ammonium
- A zinc rod is place in 0.095 M solution of zinc sulphate at 298 K, the potential
Question Type: Memory (964)
Difficulty Level: Easy (1008)
Topics: Chemical Bonding and Molecular Structure (86)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply