| ⇦ | 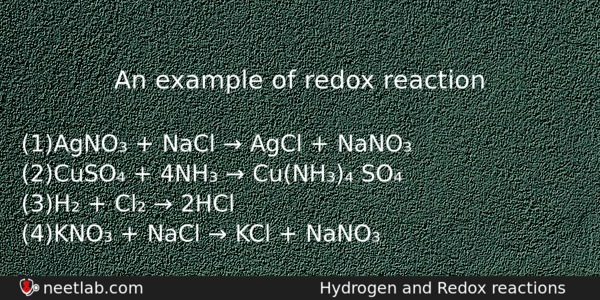
| ⇨ |
An example of redox reaction
Options
(a) AgNO₃ + NaCl → AgCl + NaNO₃
(b) CuSO₄ + 4NH₃ → Cu(NH₃)₄ SO₄
(c) H₂ + Cl₂ → 2HCl
(d) KNO₃ + NaCl → KCl + NaNO₃
Correct Answer:
H₂ + Cl₂ → 2HCl
Explanation:
H₂ + Cl₂ → 2HCl. H₂ (oxidation state = 0 ) changes to H⁺ (oxidation state = +1) and Cl₂ (oxidation state = 0) changes to Cl⁻ (oxidation state = -1). So H₂ has been oxidised and Cl₂ has been reduced, making this is redoxreaction.
Related Questions: - In the van der Waals equation, ‘a’ signifies
- An organic compound contains 49.3% carbon,6.84% hydrogen and its vapour density
- The most common lanthanide is
- An isomer of ethanol is
- The H – O – O bond angle in H₂O₂ is
Topics: Hydrogen and Redox Reactions
(174)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- In the van der Waals equation, ‘a’ signifies
- An organic compound contains 49.3% carbon,6.84% hydrogen and its vapour density
- The most common lanthanide is
- An isomer of ethanol is
- The H – O – O bond angle in H₂O₂ is
Topics: Hydrogen and Redox Reactions (174)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply