| ⇦ | 
| ⇨ |
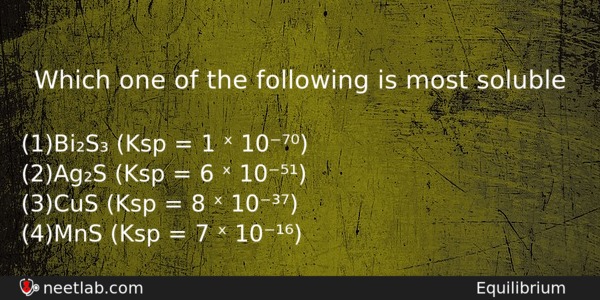
Which one of the following is most soluble
Options
(a) Bi₂S₃ (Ksp = 1 ˣ 10⁻⁷⁰)
(b) Ag₂S (Ksp = 6 ˣ 10⁻⁵¹)
(c) CuS (Ksp = 8 ˣ 10⁻³⁷)
(d) MnS (Ksp = 7 ˣ 10⁻¹⁶)
Correct Answer:
MnS (Ksp = 7 ˣ 10⁻¹⁶)
Explanation:
No explanation available. Be the first to write the explanation for this question by commenting below.
Related Questions: - The general molecular formula for a cycloalkyl group is
- The ionic radii of isoelectronic species N³⁻, O²⁻ and F⁻ are in the order
- Among the following the paramagnetic one is
- Excess nitrate in drinking water can cause
- According to MO theory which of the following lists ranks the nitrogen species
Topics: Equilibrium
(104)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- The general molecular formula for a cycloalkyl group is
- The ionic radii of isoelectronic species N³⁻, O²⁻ and F⁻ are in the order
- Among the following the paramagnetic one is
- Excess nitrate in drinking water can cause
- According to MO theory which of the following lists ranks the nitrogen species
Topics: Equilibrium (104)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply