| ⇦ | 
| ⇨ |
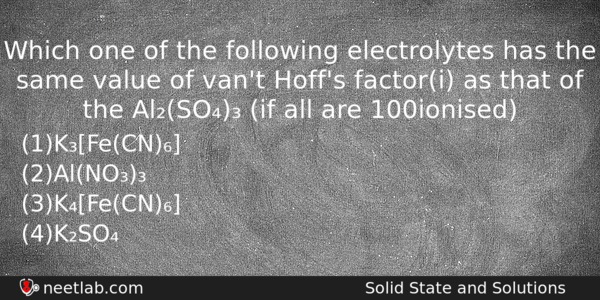
Which one of the following electrolytes has the same value of van’t Hoff’s factor(i) as that of the Al₂(SO₄)₃ (if all are 100% ionised)
Options
(a) K₃[Fe(CN)₆]
(b) Al(NO₃)₃
(c) K₄[Fe(CN)₆]
(d) K₂SO₄
Correct Answer:
K₄[Fe(CN)₆]
Explanation:
No explanation available. Be the first to write the explanation for this question by commenting below.
Related Questions: - If 200 mL of gas at 27°C is cooled to 7°C at constant pressure, its final volume will be
- In which of the following pairs, both the species are not isostructural ?
- In the reaction with HCl, an alkene reacts in accordance with the Markovnikov’s
- Amongest the following,the compound that can most readily get sulphonated is
- The reaction of an organic compound with ammonia followed by nitration
Topics: Solid State and Solutions
(91)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- If 200 mL of gas at 27°C is cooled to 7°C at constant pressure, its final volume will be
- In which of the following pairs, both the species are not isostructural ?
- In the reaction with HCl, an alkene reacts in accordance with the Markovnikov’s
- Amongest the following,the compound that can most readily get sulphonated is
- The reaction of an organic compound with ammonia followed by nitration
Topics: Solid State and Solutions (91)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply