| ⇦ | 
| ⇨ |
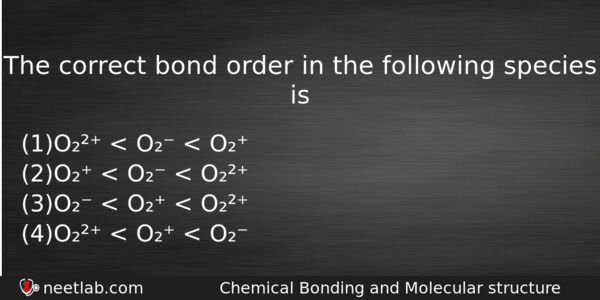
The correct bond order in the following species is
Options
(a) O₂²⁺ < O₂⁻ < O₂⁺
(b) O₂⁺ < O₂⁻ < O₂²⁺
(c) O₂⁻ < O₂⁺ < O₂²⁺
(d) O₂²⁺ < O₂⁺ < O₂⁻
Correct Answer:
O₂⁻ < O₂⁺ < O₂²⁺
Explanation:
O₂⁺ ion -Total number of electrons (16-1)=15.
Electronic configuration σ1s² < σ*1s² < σ2s² < σ*2s² < σ2p²(x) < π2p²(y)=π2p²(z)< π*2p¹(y)
Bond order =N(b)-N(a)/2 = 10-5/2 = 5/2 =2 1/2 O⁻₂ (super oxide ion): Total number of electrons (16+1)=17
Electronic configuration σ1s² < σ*1s² < σ2s² < σ*2s² < σ2p²(x) < π2p²(y)=π2p²(z)< π*2p² (y)=π*2p¹(z)
Bond order =N(b)-N(a)/2 = 10-7/2 = 3/2 =1 1/2
O₂⁺² ion :Total number of electrons (16-2)=14.
Electronic configuration σ1s² < σ*1s² < σ2s² < σ*2s² < σ2p²(x) < π2p²(y)=π2p²(z)
Bond order =N(b)-N(a)/2 = 10-4/2 = 6/2 =3
So bond order :O⁻₂ <O₂⁺<O₂²⁺
Related Questions: - Which one of the following statements concerning lanthanides elements is false
- If 1 litre of N₂ is mixed with 2 litre of O₂, quantity explaining it is
- How many mole of MNO₄⁻ ion will react with 1 mole of ferrous oxalate in acidic medium
- The correct order of electron affinity is
- The pH value of the solution in which a particular amino acid dose not migrate
Topics: Chemical Bonding and Molecular Structure
(86)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- Which one of the following statements concerning lanthanides elements is false
- If 1 litre of N₂ is mixed with 2 litre of O₂, quantity explaining it is
- How many mole of MNO₄⁻ ion will react with 1 mole of ferrous oxalate in acidic medium
- The correct order of electron affinity is
- The pH value of the solution in which a particular amino acid dose not migrate
Topics: Chemical Bonding and Molecular Structure (86)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply