| ⇦ | 
| ⇨ |
Which one of the following esters gets hydrolysde most easily under alkaline conditions
Options
(a)
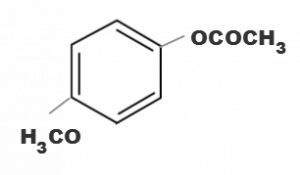
(b)
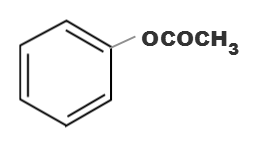
(c)
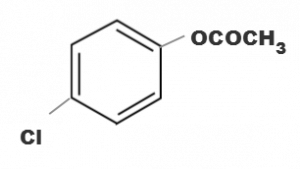
(d)
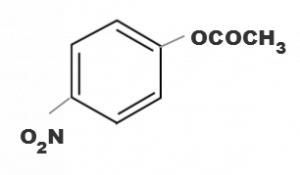
Correct Answer:
(d)

Explanation:
No explanation available. Be the first to write the explanation for this question by commenting below.
Related Questions: - Which of the following is low boiling liquid
- Which of the following elements will have the lowest first ionisation energy?
- Difference in density is the basis of
- Two possible stereo-structures of CH₃CHOHCOOH, Which are optically active are called
- Copper metal crystallizes with a face centred cubic (fcc) lattice .It has
Topics: Aldehydes Ketones and Carboxylic Acid
(89)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- Which of the following is low boiling liquid
- Which of the following elements will have the lowest first ionisation energy?
- Difference in density is the basis of
- Two possible stereo-structures of CH₃CHOHCOOH, Which are optically active are called
- Copper metal crystallizes with a face centred cubic (fcc) lattice .It has
Topics: Aldehydes Ketones and Carboxylic Acid (89)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

electron withdrawing group attatched to the benzene ring increases the reactivity towards nucleophilic substitution reaction. Since NO2 group is strong electron withdrawing group in basic medium.Hence ester containing NO2 group will be hydrolysed more easily