| ⇦ | 
| ⇨ |
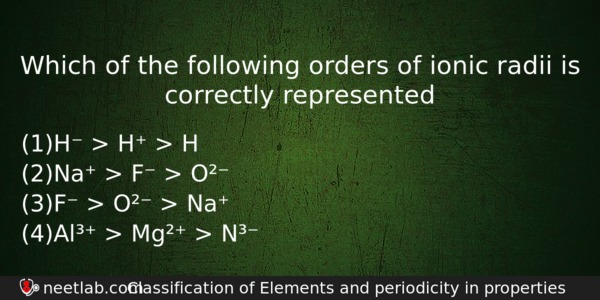
Which of the following orders of ionic radii is correctly represented
Options
(a) H⁻ > H⁺ > H
(b) Na⁺ > F⁻ > O²⁻
(c) F⁻ > O²⁻ > Na⁺
(d) Al³⁺ > Mg²⁺ > N³⁻
Correct Answer:
none
Explanation:
Cations lose electrons and are smaller in size than the parent atom whereas anions add electrons and are larger in size than the parent atom. Hence the order is H⁻ >H>H⁺.
For isoelectronic species the ionic radii decreases with increase in atomic number i.e nuclear charge.
Hence the correct orders are O²⁻>F⁻>Na⁺ and N³⁻>Mg²⁺>Al³⁺
Related Questions: - The compound used in enrichment for uranium for nuclear power plant is
- Atoms in a P₄ molecule of white phosphorus are arranged regularly in the follow
- The IUPAC name of the compound having the formula CCl₃CH₂CHO is
- Allergy is the state in which a person experiences
- The equilibrium constant of a reaction is 300. If the volume of a reaction flask
Topics: Classification of Elements and Periodicity
(94)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- The compound used in enrichment for uranium for nuclear power plant is
- Atoms in a P₄ molecule of white phosphorus are arranged regularly in the follow
- The IUPAC name of the compound having the formula CCl₃CH₂CHO is
- Allergy is the state in which a person experiences
- The equilibrium constant of a reaction is 300. If the volume of a reaction flask
Topics: Classification of Elements and Periodicity (94)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply