| ⇦ | 
| ⇨ |
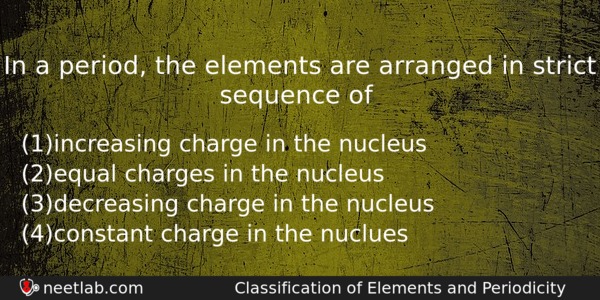
In a period, the elements are arranged in strict sequence of
Options
(a) increasing charge in the nucleus
(b) equal charges in the nucleus
(c) decreasing charge in the nucleus
(d) constant charge in the nuclues
Correct Answer:
increasing charge in the nucleus
Explanation:
No explanation available. Be the first to write the explanation for this question by commenting below.
Related Questions: - The hydrogen ion concentration in mol/dm, in a 0.2 M solution of a weak acid
- A solid compound XY has NaCl structure . If the radius of the cation is 100 pm,
- As the nuclear charge increases from neon to calcium the orbital energy
- Diazo-coupling is useful to prepare some
- When 4g of iron is burnt to ferric oxide at constant pressure, 29.28 kJ of heat
Topics: Classification of Elements and Periodicity
(94)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- The hydrogen ion concentration in mol/dm, in a 0.2 M solution of a weak acid
- A solid compound XY has NaCl structure . If the radius of the cation is 100 pm,
- As the nuclear charge increases from neon to calcium the orbital energy
- Diazo-coupling is useful to prepare some
- When 4g of iron is burnt to ferric oxide at constant pressure, 29.28 kJ of heat
Topics: Classification of Elements and Periodicity (94)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Please give explanation